Hack a Taq
Now that there are several DIY PCR machines available, the next challenge was to find a reasonable source for Taq polymerase. Also, as a side-project for the BIO-DESIGN for the REAL WORLD at EPFL, we wanted to see if we can make the "wet" part of DIY PCR more accessible, so that PCR could be used as a coliform bacteria detection method from water samples.
PLEASE NOTE that to use genetically modified bacteria, you will need to check and comply with your local/national regulations,
which can be found here for Switzerland: BAG and here
This is a happy confluence of several items on the to-do list, and the process will be documented on biodesign.cc.
EDITING IN PROGRESS!!
This is the pre-hack-a-taq documentation. Once the session is over, missing details will be added.
Objectives
1. test the Wild OpenPCR machine (gaudilabs)
2. hack the Taq isolation protocol from Open Biotechnology, and see if we can make and purify the enzyme with alternative reagents from the consumable reagents sold there (hackteria open source bioart...)
3. test out some PCR primers to detect E. coli (biodesign for the real world)
Techniques
- PCR
- agarose gel analysis of PCR products
- DNA extraction from E. coli
- Taq protein extraction
- IDEXX coliform / e.coli detection
You will need
We will try to make this list modular, so that you can do each part independently.
Equipment for
agarose gel electrophoresis
- agarose gel box (Urs)
- weighing scale
- measuring cup for volume measurements (preferably metric system)
- pipettes
- power source
- camera
PCR
- PCR conventional machine
- "Wild OpenPCR" machine (Urs)
- "My Personal OpenPCR" machine
- pipettes
| Conventional PCR | Wild OpenPCR | My Personal OpenPCR |
|---|
Taq protein production and extraction
- heatable water bath (or a pot on a stove)
- water boiler/pot
- thermometer
- vortex
- magnetic stir plate
- magnet
- shaker at 37C
- an erlenmeyer flask
- a bottle with not-so-wide neck that can resist pressure cooking
- bunsen burner/ spirit lamp (for bench sterile technique)
- pressure cooker (for media sterilization)
- electric or gas stove/cooking surface
- weighing scale
- desk top centrifuge (Urs)
Consumable materials
- bacterial culture tubes
- PCR tubes
- pipette tips
- cryotubes
Reagents
Bacterial Transformation
- LB media
- competent host bacteria (E. coli Rosetta strain for protein production after IPTG induction )
PCR
- master mix
- PCR grade water
- dNTP
- MgCl2
- commercial Taq
- buffer concentrate
- forward and reverse primers - we discuss the background on why we first chose some primers for the tuf gene on biodesign.cc.
- Lyophilized Taq mix (no cold chain needed)
Protein Production
- LB media
- 2xYT media (this link explains the different types of media, and their applications)
- 1mM IPTG
- chloramphenicol (34ug/ml final)
- ampicillin (100ug/ml final)
Protein Extraction
- Open Biotech Lysis Buffer
- lysozyme
- CaCl2
- DNAse
- Ammonium Sulfate
Agarose Gel Electrophoresis
- Nile Blue or methylene blue for visualizing DNA bands
- 100bp DNA Ladder (size reference)
- loading buffer (to make the sample settle in the well)
- running buffer
Making Bacterial Stocks
50% glycerol sterile
Protocols
From Open Biotechnology
Isolation of Taq expressed in E. coli
We will be comparing the pOpenTaq protocol and the protein extraction kit protocol provided by Open Biotechnology, with another protocol provided by the protein expression core facility at EPFL:
Taq_polymerase_isolation_from_E_coli.pdf
Colony PCR for E. coli genomic sequences
Boiling miniprep protocol = add water and boil
- from the bio.net thread from 1997
Or, just drop the bacteria/colony into the PCR tube and start the PCR
Also take a look at this well-documented resource Isolation and Purification of Total Genomic DNA from Gram-Negative Bacteria.
Typical PCR conditions with Taq
Here is a place to start from the NEB site.
Agarose Gel Electrophoresis
Nice protocol on Addgene.
We tried staining gels with methylene blue - this is a safer dye (MSDS) than DNA intercalating dyes. The concentration and staining time needs a bit more adjustment to get a better signal to noise ratio. You can save the solution and re-use it many times.
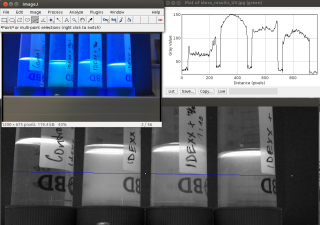
IDEXX Coliform detection
We tested the Colilert®-18 from IDEXX. The reagent we received loooong ago from some peeps at EAWAG. Diluted some samples of Rosetta with PBS, various dilutions 1, 1:20, 1:400, control PBS. Incubated in a cardboard box above the hot-water piping in the hallway overnight. Nothing turned yellow.... yet....
Results
"incubated" a bit longer... at various temperatures for ca 40h. got positiv coliform detection at higher concentrations. also positive for e.coli in UV light.
Notes on PCR primer Design
PCR primer design has great rules of thumb, but also helps to have experience and help with empirical testing. These sites are very good resources:
- University of Ontario, CA has nicely organized molecular analysis links, this page specific for PCR primer design, including NCBI's primer blast page
- European Molecular Biology Laboratory (EMBL) is a great resource for nearly everything in contemporary biological research --- this is their primer design page
For the BIO-DESIGN for the REAL WORLD project, we decided to use a primer pair designed and tested by Maheux et al. Water Research 43 (2009) pp. 3019-3028. This primer pair detects the tuf gene -and according to the paper, is quite specific for detecting E. coli and Shigella:
TEcol553 5′-TGG GAA GCG AAA ATC CTG-3′
TEcol754 5′-CAG TAC AGG TAG ACT TCT G-3′
- more about the musings on the selection process on biodesign.cc.
Details
What to bring, Where to stay
For the Hack-a-Taq weekends, we will be preparing materials outlined above.
The idea is for you to read what is on this page, get inspired, and do bring your own ideas and materials for the session!
Come for parts of the day, come to all the days.
If you are coming from out of town, it would be great to know which nights you will need accommodations. It would be best if you can bring a camping mattress and a sleeping bag in addition to change of clothes and toiletries. Please contact Sachiko to set up arrangements: sachiko a t biodesign.cc
Dates and Times and Meeting Points
- 5 April - Optional pre-workshop day at EPFL
- 14:00pm (EPFL SV3.615) meet and greet.
- 18:00 pm (EPFL BM5202) PUBLIC THESIS DEFENSE - Highthroughput 3D culture and analysis of stem cell differentiation (room and official title coming soon)
- 6 April
- 11:00 am (place de la Ripponne, steps of palace de rumine) OUTFITTING THE LAB shopping in the Riponne flea market -
- We are running late this morning - for any questions, please contact Sachiko (at) biodesign.cc
- 7 April
- 11:00 am (m1 metro, Flon)
NOW CANCELLED- 13 APRIL SESSION
- 12 April - Optional pre-workshop day at EPFL
- 12:15pm (EPFL SV3.615) meet the students of BIO-DESIGN for the REAL WORLD.
- 13 April
- 10:00 am (place de la Ripponne, steps of palace de rumine) OUTFITTING THE LAB shopping in the Riponne flea market
New Hackteria GelBox
During the pre-Hack-a-Taq night on thursday, we went to FabLab Luzern to make some gadgets to be used in the lab.
HiroKouri Gel Carrier Souvernir
Hackteria GelBox v0.1
Testing the GelBox
We gave it a try using GeneRuler 100bp. To get more Voltage we connected 2 powersupplies in series. Let it run for ca 50 min and then stained with Methylene Blue overnight. Seems we overstained it quite lot, but washing out the color from the gel makes the ladder visible again. Also seems we ran it too far (long).